NICA Accreditation Program Director Edie Gigot, MSN, RN, MBA explains the importance of established standards in healthcare and expands on the National Infusion Center Association’s Standards of Excellence for Ambulatory Infusion Centers. Edie also explores the application of these standards in infusion center accreditation, discussing the why, who, and how of becoming a NICA-accredited center.
Why do we need standards of care?
Most healthcare providers and consumers are aware of the importance of having standards of care. Standards of care are required and necessary for healthcare professionals to ensure we provide ethical, high-quality, safe, and consistent care to patients. It’s also important to understand that standards of care provide the basis for our clinical practice guidelines and…
- Help to reduce unwarranted variation, as they identify and define the care people should expect to receive regardless of the care setting;
- Establish the benchmark that determines whether our professional and ethical obligations to patients have been met;
- Provide a reliable measure for accountability, validation assessments, training, and educational opportunities;
- Help identify gaps in existing systems and processes; and,
- Support the achievement of better patient outcomes, resulting in the delivery of higher-value care that benefits patients, personnel, and industry partners.
What standards should you follow?
W. Edwards Deming said it best, “Everyone doing their best is not the answer. It’s necessary that people know what to do.”
Understanding how and where the standards of care we aim to adhere to come from is equally important as understanding their purpose. Most people don’t realize that anyone can write something down and call them “standards”; in other words, there are no “standards police.”
There is currently no single regulatory body overseeing all infusion centers on a national scale, leaving providers to look for ways to demonstrate they are delivering safe, consistent, high-quality care. As the national trade association, the National Infusion Center Association (NICA) developed the first comprehensive set of standards for this setting to be recognized by the American National Standards Institute (ANSI).
As part of the NICA standards development process, some of the key requirements included:
- Consensus by a “consensus body” that included representatives from materially affected and interested parties, including representatives from interest categories throughout the industry and professions that included medical doctors, nurse practitioners, physician assistants, pharmacists, registered nurses, licensed practical nurses, and administrative roles.
- A broad-based public review and comment on the draft standards, which included NICA ensuring a period in which open public comment was allowed, and any feedback received was considered in the standards development process.
- The right to appeal if any standards development participants believed that the due process principles were not sufficiently respected during the standards development process and a follow-up process in which NICA ensured consideration and response if any appeals had been received.
So, if we have standards of care, why do we need accreditation?
Accreditation is a voluntary process that determines an organization’s technical competence and is used to verify compliance with established standards. In addition, accreditation…
- Is an evaluation tool that includes a process in which trained external reviewers evaluate an organization’s compliance with established standards, laws, and regulatory requirements
- Focuses on continuous improvement strategies and achievement of optimal quality
- Provides a framework to create and implement processes that improve operational effectiveness and outcomes and help to drive strategic direction within an organization
- Establishes industry benchmarks for quality, safety, and accountability
- Provides the public assurance that the care and services provided meet acceptable levels of established quality and excellence
- Reduces the disparities in the quality of care and safety across care settings
What sets the NICA Standards and Accreditation apart from others?
The NICA Standards were created by members of the infusion center industry for the infusion center industry. In reviewing the NICA Standards compared to other industry standards, it’s easy to identify some of the key differences.
Infusion-Related Reaction Management
Other accreditation standards require the basics, such as policies for the use of emergency medication, staff education, and patient monitoring; however, unlike the NICA Standards, which cover all of this and more, these other standards do not provide a comprehensive list of the medication and supplies that must be available, the type of frequency and monitoring best for patients to ensure the safest delivery of care, or how often to document emergency and reaction management interventions effectively.
Medication Administration
Other accreditation standards require policies and procedures for administering medications, patient monitoring, and personnel training, but what about the guidance on what that means when administering infusible and injectable medications? The NICA Standards provide considerable detail in regard to medication administration, including a grouping of 81 standards that identify best practices for areas such as:
- Preparation and administration of infusible and injectable medications
- Valid medication orders, including all required adjunct orders such as flush and reaction management
- Baseline and ongoing patient assessment, observation, and monitoring requirements
- Infusion rate management and titration guidance
- Infusion pump use, management, and training
- Residual volume management and waste reduction
- Clinical documentation for infusible medications and vascular access-related activities
Environment of Care
Other accreditation standards state organizations must have a treatment care area and a waiting area, but this leaves organizations searching for answers on how to set up their infusion suites/centers and what layouts and amenities are best practices for delivering safe infusion care. The NICA Standards provide detailed guidance that includes acceptable treatment area structure and set-up, including best practices for patient waiting areas and patient care area requirements.
These requirements include patient equipment, nursing stations, call systems, adequate space for patients to have guests, comfortable temperature controls, and safe walking paths. The NICA Standards also provide specific guidance for adequate spacing between infusion chairs to allow for resuscitation activities and equipment, if ever required, and the set-up of clinician workspaces to support efficiency and safety.
It’s important to remember it’s our responsibility to keep patients safe; not being properly prepared for safe medication administration puts both patients and personnel at risk.
What accreditation program should you choose?
To sum up the relationship between the NICA Standards of Excellence for Ambulatory Infusion Centers and the NICA Accreditation Program, the standards establish the safety and quality requirements – they’re the roadmap for what it is we need to do to ensure we’re operating in the safest manner possible, providing the safest care while helping to ensure quality outcomes for all stakeholders. Having established safety and quality requirements sets the expectation for reasonable and achievable organizational performance, and the NICA Accreditation Program assesses whether or not an organization is achieving that expected performance.
How is the NICA Accreditation Program different?
The NICA Accreditation was built upon a robust, evidence-based quality framework. This framework provides the basis upon which each step of the program was developed, with the key focus and commitment to ensuring the most comprehensive, cost-effective experience for participating organizations.
NICA partners with organizations throughout their accreditation journey, assigning an Accreditation Program Navigator to each organization to help ensure their ongoing support and success. In addition, NICA provides participating organizations with a flexible timeline and upfront self-evaluation (e.g., gap analysis) opportunity to support the identification of areas of improvement so that thoughtful and focused attention can occur from the start.
NICA Accreditation positions organizations for success amidst rapid market changes by creating a standardized care delivery foundation focused on consistent, high-value, cost-effective care. The NICA Accreditation Program aims to improve consistency in the quality and safety across infusion enterprises, protect patients by providing objective measurement of high-quality infusion care, and promote awareness by highlighting NICA-accredited organizations as Ambulatory Infusion Centers of Excellence (AICE) to payers, patients, and referring prescribers.
As of October 2023, the NICA Accreditation Program has partnered with RxToolKit to promote the accessibility of safe infusion therapy and compliance with NICA’s standards. Through RxToolKit’s clinical training platform, infusion center teams have access to innovative educational content, infusion-specific continuing education courses, and more to assist practices in standard compliance. RxToolKit’s software also provides comprehensive medication information and drug-specific calculators for ease-of-access at the point of care, enhancing safety and clinical competency within the industry.
How do you become NICA-accredited?
The NICA Accreditation begins with the application process. Organizations must begin by applying to the program and meeting the following eligibility requirements:
- Identify as one of the following infusion center types: freestanding, hospital-based, office-based, or pharmacy-based
- Located and operating within the United States (US) and/or US territories
- Established and implemented policies and procedures
- Have at least one dedicated facility/site in which infusion services are provided
- Licensed and compliant with all applicable federal, state, and local laws and regulations
Once an organization completes the NICA Accreditation application process and is deemed eligible to participate, the next steps in the process begin. The image below provides a high-level overview of the complete NICA Accreditation Program process.
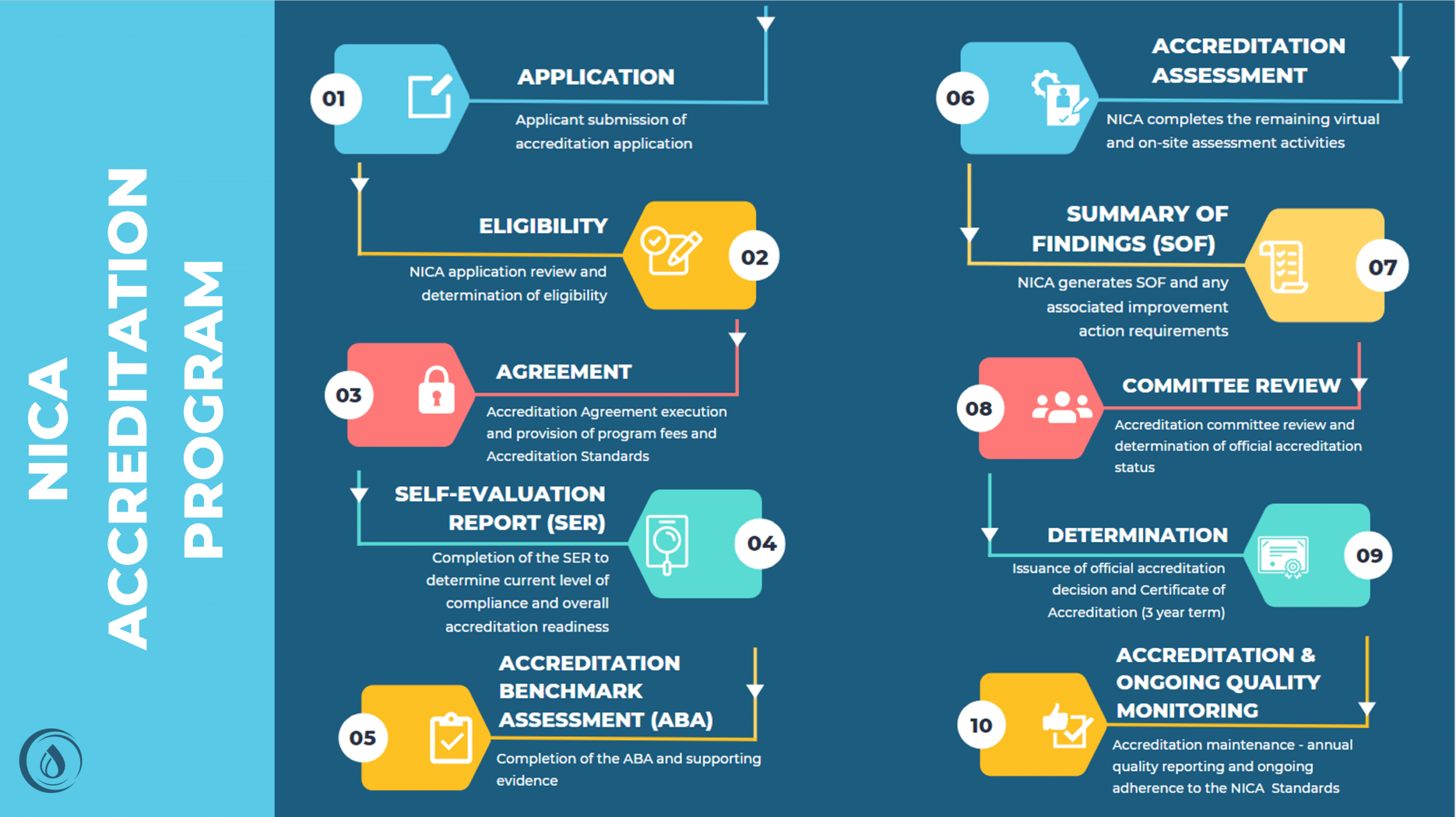
Organizations that achieve NICA Accreditation are deemed AICE. This achievement exemplifies an organization’s commitment to excellence through the delivery of high-quality, value-based infusion services. In addition, it proactively positions organizations ahead of the competition as payers trend toward requiring accreditation across their network of infusion providers.
Organizations interested in becoming an AICE will further be differentiated as they join NICA in the ongoing quest to build an Infusion Center of Excellence network across the country, driving best practices, promoting quality standards and excellence in care delivery, and further positioning the delivery channel to navigate future challenges and threats.






