In this article, Dr. Jill Paslier, PharmD, CSP, FISMP analyzes the benefits of medication error surveillance and how it can enhance patient safety and improve clinical outcomes. Dr. Paslier provides three key components to implementing a successful MES program, and highlights the probable outcomes of increased monitoring.
Introduction to Medication Error Surveillance
Medication error surveillance (MES), also referred to as “medication error monitoring,” is a proactive approach by healthcare administrators to actively screen for events indicating potential medication errors throughout the medication use process. It involves vigilant monitoring and identification of incidents that may compromise patient safety. This surveillance is important across various healthcare settings, including hospitals; infusion centers; and specialty, infusion, and community pharmacy settings.
The National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) defines medication errors as:
Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing, order communication, product labeling, packaging, and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use.1
Errors in medication use are challenging to eliminate completely, especially in environments where human interaction plays a role. Some pharmacies and health systems lack active surveillance programs to detect such errors, instead relying on patient-reported incidents or chance discoveries. This passive approach often leads to underreporting and missed opportunities for timely intervention.
As emphasized in the landmark publication To Err Is Human, errors are inherent to human nature, and proactive monitoring is crucial for minimizing their impact and ensuring patient safety throughout the medication use process.2
Benefits of a Robust MES Program
A robust medication error surveillance program offers several key benefits that contribute to enhancing patient safety and improving healthcare outcomes.
Early Detection
Early detection is paramount. By actively monitoring for errors, healthcare providers are more likely to catch them early. This allows for timely intervention to prevent patient harm, even if an error has already occurred.
Promoting Improvements
Additionally, such programs foster a culture of continuous improvement within healthcare organizations. Error reporting and analysis enable the identification of root causes, empowering providers to implement systemic changes and high-leverage strategies to reduce the likelihood of errors recurring.
Patient Safety
Ultimately, the most significant benefit is the enhancement of patient safety. A proactive approach to medication error surveillance ensures a safer healthcare and medication experience for patients, thereby reducing the risk of adverse events and improving overall outcomes.2
How to Implement an MES Program
When establishing a medication error surveillance program, it’s essential to incorporate various surveillance activities. Examples of these activities include double checks, monitoring for adverse drug events (ADEs) or other triggers, and patient counseling. Assess which of these activities align with your organization’s workflow, determine how you’ll implement them, and establish the frequency at which they’ll be conducted.
Double Checks
Double checks involve two qualified individuals independently verifying medication-related tasks or processes to ensure accuracy and reduce the risk of errors. This verification process adds an extra layer of scrutiny and commonly occurs during medication dispensing, administration, and preparation, especially for high-risk medications or complex procedures.
For instance, pharmacists in pharmacy settings may conduct double checks when verifying prescription orders or compounding medications, while nurses in healthcare settings may perform double checks when administering medications to patients by confirming the medication, dosage, and patient identification.
Implementing double checks aligns with best practices for medication safety endorsed by organizations such as the Institute for Safe Medication Practices (ISMP), which advocates for systematic approaches to error prevention and risk mitigation in healthcare settings.3-5
While not all states enforce double-checking practices, pharmacies can still benefit from adopting these processes, particularly for medications prone to frequent errors or classified as high-alert by ISMP. High-alert medications, such as chemotherapy drugs and neuromuscular blocking agents, pose a heightened risk of significant patient harm if used in error, warranting additional verification measures. ISMP provides a comprehensive list of high-alert medications in the Acute Care4 and Community Pharmacy5 settings and recommendations for safer use, guiding pharmacies in implementing effective double-checking practices.
Monitoring for Adverse Events and Other Triggers
Another form of surveillance involves monitoring for adverse events or triggers to identify potential medication errors. These triggers may or may not be directly linked to medication errors, but could indicate issues worth investigating. Examples include ADEs, unexpected changes in vital signs, readmission rates, laboratory abnormalities, the use of reversal agents, and patient complaints or patient reported errors.
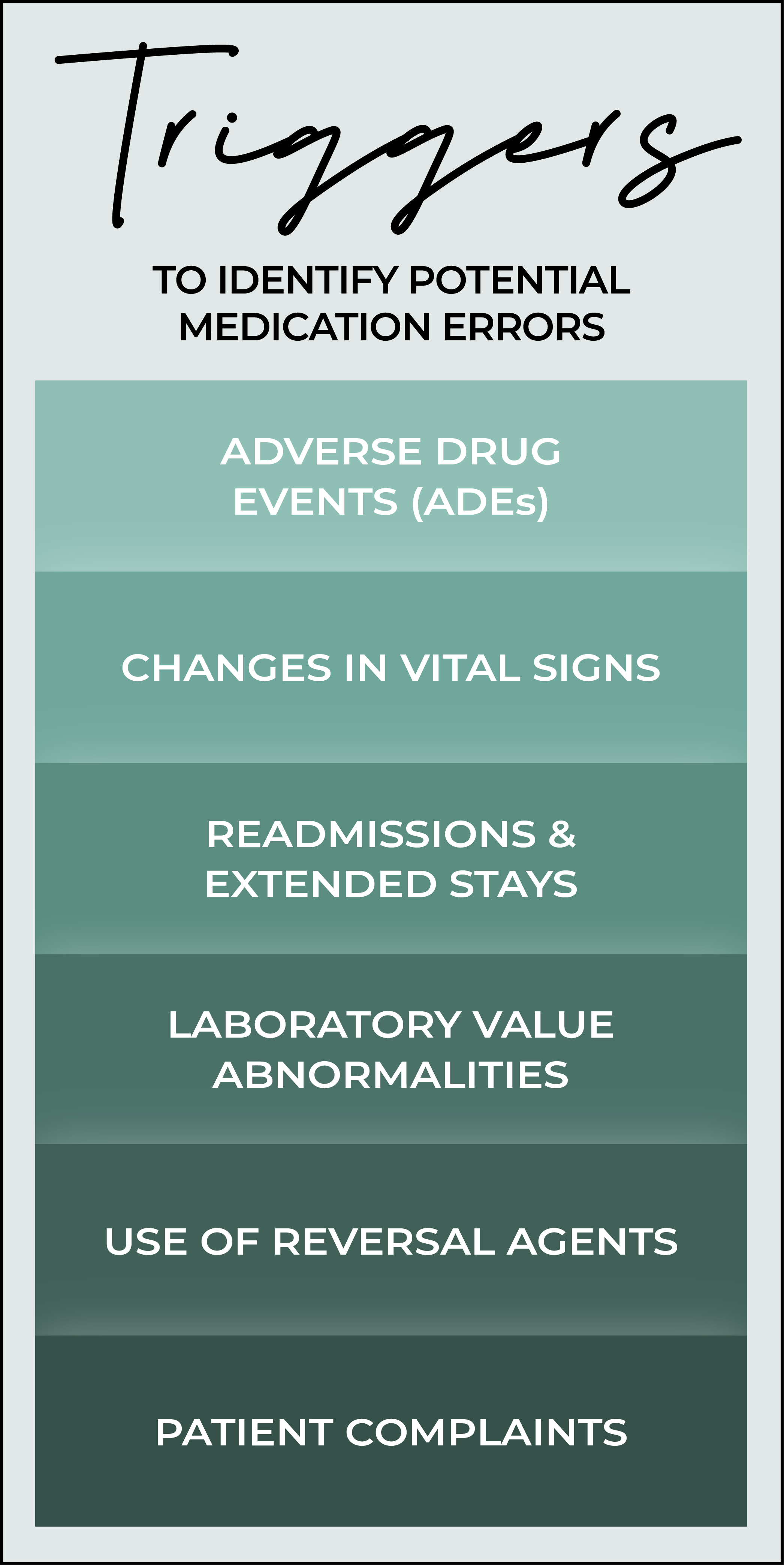
Adverse Drug Events (ADEs)
ADEs are unintended and harmful events associated with medication use. While some ADEs result from patients having a negative reaction to a medication, others may stem from inappropriate medication use or errors in medication management.2,3
Changes in Vital Signs
Unexpected changes in a patient’s vital signs during medication administration, especially in the infusion setting, can indicate potential adverse reactions or medication errors.2,3 For example:
- Sudden Hypotension: A rapid drop in blood pressure after medication infusion may suggest an adverse reaction or incorrect dosage. This could result from errors like administering the wrong medication or an incorrect infusion rate.
- Tachycardia or Bradycardia: Significant deviations from the patient’s normal heart rate during or after infusion therapy may signal medication-related issues such as an ADE, overdose, or inappropriate medication use.
- Respiratory Distress: Symptoms like shallow breathing, coughing, or wheezing following medication administration–especially in outpatient infusion settings–could indicate adverse reactions or medication errors. Respiratory depression may indicate opioid overdose or incorrect opioid administration, requiring immediate intervention.
Readmissions and Extended Stays
Readmissions, extended hospital stays, or transfers to higher levels of care could result from complications related to medication, including incorrect medication usage.6,7
Laboratory Value Abnormalities
Laboratory value abnormalities refer to unexpected changes in laboratory test results that can be linked to medication use, signaling potential medication-related issues.2,7 For example:
- Elevated Liver Enzymes: Increased levels of liver enzymes may indicate liver injury caused by medications like antibiotics or immunosuppressants.
- Renal Function Decline: Decreases in renal function markers, such as elevated creatinine levels, may suggest kidney damage from nephrotoxic medications such as vancomycin.
- Abnormal Coagulation Parameters: Changes in coagulation parameters, like elevated international normalized ratio (INR), may indicate over-anticoagulation or a warfarin dose that was too high.
Use of Reversal Agents
The use of reversal agents involves administering medications to counteract the effects of other medications, which may signal an error with the initial medication.6,7 For example:
- In the case of an anticoagulant overdose, a patient receives an excessive dose of a blood thinner, leading to bleeding complications. Healthcare providers administer a reversal agent to counteract the effects of the initial dosing error.
- In an opioid overdose scenario during pain management infusion therapy, a patient receives an excessive amount of opioid medication, resulting in respiratory depression. Naloxone is administered to reverse the overdose, suggesting an error in opioid dosing.
Patient Complaints
Patient complaints refer to instances where patients report experiencing unusual symptoms or expressing concerns regarding medication. For example:
- A patient might report side effects following an intravenous (IV) infusion, suggesting a possible adverse reaction to the medication, errors in product preparation or selection, or incorrect administration techniques.
- A patient may voice concerns about potential medication errors if they suspect they were given the wrong medication, incorrect dosage, strength, instructions, quantity, and so forth.2,5
Patient Counseling
Engaging patients in surveillance activities involves educating them about potential medication errors and empowering them to recognize and report any suspected incidents. In the infusion setting, pharmacists or nurses may provide comprehensive counseling to patients and caregivers on medication administration techniques, potential side effects, and steps to take in case of adverse reactions. This patient counseling approach encourages active involvement in medication safety and enhances the detection and reporting of medication errors.3,5
The Outcomes of Increased Medication Error Surveillance
Enhancing medication error surveillance activities may lead to an increase in reported errors, which is actually a positive outcome. This increase signifies that more errors are being identified, allowing for proactive intervention to prevent potential harm to patients.
Rather than focusing solely on the volume of error reports, the key lies in analyzing these reports to uncover root causes and implementing systemic changes to mitigate risks in the future. This process involves conducting thorough root cause analyses and leveraging the insights gained to improve medication use systems and prevent errors from occurring again.
It’s important to understand that the success of reporting programs isn’t solely measured by the number of reports generated. Instead, the emphasis should be on using the information gathered to identify weaknesses in medication use systems and implement corrective measures. By continually monitoring and investigating medication errors, healthcare organizations can refine their processes, ultimately enhancing patient safety and optimizing healthcare outcomes.
In conclusion, medication error surveillance is crucial across various healthcare settings, serving as a cornerstone for improving patient safety, fostering quality improvement, and achieving optimal healthcare outcomes.2,7,8
1 “About Medication Errors,” National Coordinating Council for Medication Error Reporting and Prevention, 2024. [Online]. Available: https://www.nccmerp.org/about-medication-errors. [Accessed 19 February 2024].
2 L. T. Kohn, J. M. Corrigan and M. S. Donaldson, To Err is Human, Washington (DC): National Academies Press (US), 2000.
3 “Medication Safety Self Assessment® for Hospitals,” 1 April 2011. [Online]. Available: https://www.ismp.org/assessments/hospitals. [Accessed 19 February 2024].
4 “High-Alert Medications in Acute Care Settings,” 10 January 2024. [Online]. Available: https://www.ismp.org/recommendations/high-alert-medications-acute-list. [Accessed 19 February 2024].
5 “High-Alert Medications in Community/Ambulatory Care Settings,” 30 September 2021. [Online]. Available: https://www.ismp.org/recommendations/high-alert-medications-community-ambulatory-list. [Accessed 19 February 2024].
6 “Triggers and Trigger Tools,” Agency for Healthcare Research and Quality, 7 September 2019. [Online]. Available: https://psnet.ahrq.gov/primer/triggers-and-trigger-tools. [Accessed 19 February 2024].
7 “Pump Up the Volume: How to Prioritize Events and Analyze Error Data,” Institute for Safe Medication Practices, 9 February 2023. [Online]. Available: https://www.ismp.org/resources/pump-volume-how-prioritize-events-and-analyze-error-data. [Accessed 19 February 2024].
8 “Statement on Medication Error Rates,” National Coordinating Council for Medication Error Reporting and Prevention, 24 June 2008. [Online]. Available: https://www.nccmerp.org/statement-medication-error-rates. [Accessed 19 February 2024].





